Draw the sun and label the 4 layers from middle to the surface.
Answers
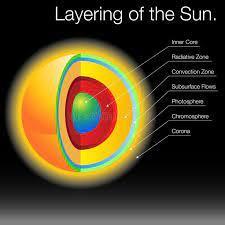
The layers of the sun can be seen in the image attached.
What are the layers of the sun?The sun is composed of several layers, including:
Core: The innermost layer of the sun where nuclear fusion takes place. The temperature in the core is about 15 million degrees Celsius.
Radiative Zone: This layer is between the core and the convection zone. Energy produced in the core is transported through the radiative zone by photons.
Convection Zone: The outermost layer of the sun's interior where hot gas rises and cooler gas sinks. The energy produced in the core is carried to the surface by convection.
Photosphere: The visible surface of the sun where most of the sun's light is emitted. The temperature of the photosphere is around 5,500 degrees Celsius.
Chromosphere: A thin layer above the photosphere that emits a reddish glow during solar eclipses. The temperature of the chromosphere ranges from 4,000 to 10,000 degrees Celsius.
Corona: The outermost layer of the sun's atmosphere, extending millions of kilometers into space. The temperature of the corona is extremely high, around 1 to 3 million degrees Celsius.
Learn more about the sun:https://brainly.com/question/17376727
#SPJ1
Related Questions
is the term used to describe the loss of a hydrogen and a halogen from an alkyl halide. the product of the reaction is a(n) .multiple choice question.addition; alkyl halidedehydrohalogenation; alkaneaddition; alkenedehydrohalogenation; alkene
Answers
Dehydrohalogenation is the term used to describe the loss of a hydrogen and a halogen from an alkyl halide. The product of the reaction is an alkene.
The term used to describe the loss of a hydrogen and a halogen from an alkyl halide is dehydrohalogenation. The product of the reaction is an alkene. Dehydrohalogenation is a type of organic reaction in which a hydrogen halide (HX) is removed from an organic molecule, typically an alkyl halide, to produce an alkene.
Alkyl halides are a type of organic compound in which one or more halogen atoms, such as chlorine or bromine, are substituted for hydrogen atoms on an alkane chain. The general formula for an alkyl halide is RX, where R is an alkane chain and X is a halogen.The dehydrohalogenation of an alkyl halide produces an alkene and a hydrogen halide, such as HCl or HBr. The reaction is catalyzed by a strong base, such as sodium ethoxide or potassium tert-butoxide. The mechanism of the reaction involves the removal of a proton from the alkyl halide by the base, followed by the elimination of the halide ion to produce an alkene.
For more such questions on Dehydrohalogenation , Visit:
https://brainly.com/question/14787332
#SPJ11
A mixture of 90. 0grams of ch4 and 10. Ograms of argon has a pressure of 250 torr under the condition of constant temperature and pressure the partial pressure of ch4 is
Answers
The partial pressure of CH₄ in the mixture is 239 torr.
We can use the mole fraction of methane (CH4) to calculate its partial pressure in the mixture. First, we need to convert the masses of each component into moles:
moles of CH₄ = 90.0 g / 16.04 g/mol = 5.61 mol
moles of Ar = 10.0 g / 39.95 g/mol = 0.250 mol
Next, we can calculate the total moles of gas in the mixture,
total moles = moles of CH₄ + moles of Ar = 5.61 mol + 0.250 mol = 5.86 mol
Now we can calculate the mole fraction of CH₄,
mole fraction of CH₄ = moles of CH₄ / total moles = 5.61 mol / 5.86 mol = 0.957
Finally, we can use the mole fraction and total pressure to calculate the partial pressure of CH₄,
partial pressure of CH₄ = mole fraction of CH₄ x total pressure = 0.957 x 250 torr = 239 torr
To know more about mixture, here
brainly.com/question/20853110
#SPJ4
you are given a sample of kool-aid with an unknown concentration and you measure the absorbance in the spectrophotometer. the absorbance reading is 1.5. approximately what is the concentration of the sample?
Answers
Answer: The absorbance reading in this case was 1.5, and using the Beer-Lambert law and the extinction coefficient of the light used in the spectrophotometer, the concentration of the sample was approximately 1.5.
The concentration of a sample of Kool-Aid can be determined by measuring its absorbance in a spectrophotometer. In this case, the absorbance reading was 1.5.
To calculate the concentration of the sample, we must first understand how absorbance is related to concentration. The Beer-Lambert law states that the absorbance of a sample is directly proportional to the concentration of the sample. This means that the higher the concentration, the higher the absorbance, and vice versa.
Therefore, to find the concentration of the sample given its absorbance reading of 1.5, we can use the following equation: Concentration = Absorbance/Extinction Coefficient.
The extinction coefficient is a constant,and can be determined from the wavelength of the light used in the spectrophotometer.
Once we have determined the extinction coefficient, we can calculate the concentration of the sample. Plugging in the absorbance and extinction coefficient into the equation gives us the concentration of the sample. In this case, approximately 1.5.
In summary, the concentration of a sample of Kool-Aid can be determined by measuring its absorbance in a spectrophotometer. The absorbance reading in this case was 1.5, and using the Beer-Lambert law and the extinction coefficient of the light used in the spectrophotometer, the concentration of the sample was approximately 1.5.
Learn more about the Beer-Lambert law here:
https://brainly.com/question/30404288#
#SPJ11
a quantity of electric charge deposits 0.732 g of ag(s) from an aqueous solution of silver nitrate. when that same quantity of charge is passed through a solution of a gold salt, 0.446 g of au(s) is formed. what is the oxidation state of the gold ion in the salt?
Answers
According to the second law of Faraday, the oxidation number of gold ions is +3.
What is the second law of Faraday?The second law of Faraday is also known as Faraday's law of electrolysis. According to this, the quantity of a substance that is deposited or released during electrolysis is directly proportional to the amount of electric charge that is transported through the electrolyte.
Given information,
Mass of silver (Ag) deposited = 0.732 g
Mass of gold (Au) deposited = 0.446 g
According to this law,
Weight of Ag/Equivalent weight of Ag = Weight of Au/Equivalent weight of Au
0.732/108 = 0.446/196.96 × valency
Since the equivalent weight of Ag is 108g and the equivalent weight of Au is 196.96g.
0.0067 = 0.0022 × valency
Valency = 0.0067/ 0.0022
Valency = 3
Therefore, the oxidation state of the gold ion (Au⁺³) is +3.
Learn more about Faraday's law, here:
https://brainly.com/question/1640558
#SPJ6
based on your melting points and demo tlc, comment on the success of the extraction experiment. are the tlc and melting points in agreement? which is the purest solid of the three? does the result make sense? explain your answer. (
Answers
If we assume that the melting points and TLC are in agreement, then we can use them to determine the purity of the solids.
The purest solid would have the highest melting point and the most distinct TLC spot. We can compare the values to ascertain which solid is the purest if the melting points and TLC are in agreement. It may be a sign that the extraction was unsuccessful or that there were impurities in the sample if there is a significant difference between the melting points or the spots on the TLC.
It's crucial to remember that melting points and TLC are not always accurate indications of purity because other variables can influence them. However, they can be a helpful tool for determining the success of an extraction experiment if the values are consistent and in agreement.
Learn more about melting point
brainly.com/question/40140
#SPJ4
a substance is maintained at a pressure of 300 x 105 [n/m^2] (absolute). which answer best describes that pressure in customary us units?
Answers
To convert the pressure from SI units (Pascals) to customary US units, we can use the following conversion factors[tex]1 Pa = 1 N/m^21 atm = 101325 Pa1 psi = 6895 Pa[/tex]
First, let's convert the pressure from Pascals to atmospheres (atm):
1 atm = 101325 Pa
[tex]300 x 10^5 Pa / 101325 Pa/atm = 2.96 atm[/tex]
Now, we can convert the pressure from atmospheres to pounds per square inch (psi):
1 psi = 0.06895 atm
[tex]2.96 atm x 0.06895 psi/atm = 0.2045 psi[/tex]
Therefore, the pressure of 300 x 10^5 N/m^2 (absolute) is approximately 0.2045 psi in customary US units.
learn more about pressure here:
https://brainly.com/question/12971272
#SPJ4
prior knowledge questions (do these before using the gizmo.) what important gas do we take in when we breathe?
Answers
Answer: The important gas that we inhale when we breathe is oxygen (O2).
It is necessary for the process of respiration. Respiration is a vital process that takes place in all living cells, including human cells. In this process, glucose (sugar) and oxygen are converted into energy (ATP), carbon dioxide (CO2), and water (H2O).
During the process of inhalation, the air enters the body through the mouth and nose. Afterward, it moves down the trachea and then into the lungs. Once inside the lungs, oxygen molecules pass through the thin walls of the capillaries and into the bloodstream, where it is transported to the rest of the body. Oxygen is essential for the proper functioning of the body.
It is used by the cells to produce energy, which is used to power various biological processes. Without oxygen, our cells would not be able to function, and we would die.
Learn more about oxygen here:
https://brainly.com/question/13905823#
#SPJ11
in the combustion analysis of 17.1 g of sugar (c12h 22o 11), what mass, in grams, of o 2 would be consumed?
Answers
In the combustion analysis of 17.1 g of sugar (C12H22O11), the mass of O2 consumed is equal to 8.55 g.
This is due to the fact that the balanced equation for the combustion of sugar is C12H22O11 + 12 O2 --> 12 CO2 + 11 H2O.
This means that for every one mole of sugar that is combusted, 12 moles of O2 are needed.
To calculate the mass of O2 consumed, the number of moles of sugar must first be calculated using the molar mass of sugar, which is 342.3 g/mol.
Therefore, 17.1 g of sugar is equal to 0.05 moles of sugar. Then, using the balanced equation, it can be seen that 0.05 moles of sugar require 0.6 moles of O2.
Finally, the mass of O2 consumed can be determined by multiplying the number of moles of O2 by the molar mass of O2, which is 32 g/mol.
Therefore, 0.6 moles of O2 is equal to 19.2 g, which is equivalent to 8.55 g of O2 consumed.
to know moremore about combustion refer here:
https://brainly.com/question/15117038#
#SPJ11
if 20.6 grams of an aqueous solution of sodium carbonate, na2co3, contains 3.11 grams of sodium carbonate, what is the percentage by mass of sodium carbonate in the solution?
Answers
Answer: The percentage by mass of sodium carbonate (Na2CO3) in an aqueous solution of sodium carbonate is 15.10%.
The percentage by mass of sodium carbonate (Na2CO3) in an aqueous solution of sodium carbonate is calculated by dividing the mass of sodium carbonate (3.11 g) by the total mass of the solution (20.6 g).
This gives us a ratio of 0.1510, which can be converted to a percentage by multiplying by 100. This results in a percentage by mass of sodium carbonate of 15.10%.
The percentage by mass of sodium carbonate can also be calculated using the following equation:
Percentage by mass of sodium carbonate = (Mass of sodium carbonate / Total mass of solution) * 100
In this case, we can substitute the known values of the mass of sodium carbonate (3.11 g) and the total mass of the solution (20.6 g) into the equation to calculate the percentage by mass of sodium carbonate in the solution.
We first divide the mass of sodium carbonate (3.11 g) by the total mass of the solution (20.6 g). This gives us a ratio of 0.1510.
We then multiply this ratio by 100 to convert it into a percentage. This gives us a percentage by mass of sodium carbonate of 15.10%.
In conclusion, the percentage by mass of sodium carbonate (Na2CO3) in an aqueous solution of sodium carbonate is 15.10%.
Learn more about percentage of chemicals here:
https://brainly.com/question/14962135#
#SPJ11
what is the relationship between the unit cell edge length a and the atomic radius r for the body-centered cubic crystal structure?
Answers
The relationship between the unit cell edge length a and the atomic radius r for the body-centered cubic (BCC) crystal structure is known as the packing factor.
The packing factor is calculated as the volume of an atom (πr3) divided by the volume of the unit cell (a3). This relationship can be expressed mathematically as:
Packing Factor = πr3 / a3
The packing factor can be used to determine the size of the unit cell for a given atomic radius. A larger atomic radius will result in a larger unit cell.
The inverse is true as well, meaning that a smaller unit cell will have a smaller atomic radius.
The BCC crystal structure is one of the most efficient packing structures, as it has a packing factor of 0.68, meaning that 68% of the unit cell volume is occupied by the atoms.
This is the highest packing factor of all the common crystal structures.
In conclusion, the relationship between the unit cell edge length a and the atomic radius r for the body-centered cubic crystal structure can be expressed as a packing factor.
The packing factor is used to calculate the size of the unit cell for a given atomic radius, and the BCC crystal structure is one of the most efficient packing structures.
to know more about packing factor refer here:
https://brainly.com/question/13439231#
#SPJ11
strain energy for alkanes interaction / compound kj/mol kcal/mol h : h eclipsing 4.0 1.0 h : ch3 eclipsing 5.8 1.4 ch3 : ch3 eclipsing 11.0 2.6 gauche butane 3.8 0.9 cyclopropane 115 27.5 cyclobutane 110 26.3 cyclopentane 26.0 6.2 cycloheptane 26.2 6.3 cyclooctane 40.5 9.7 (calculate your answer to the nearest 0.1 energy unit, and be sure to specify units, kj/mol or kcal/mol. the answer is case sensitive.)
Answers
The strain energy (in kJ/mol and kcal/mol) for various alkane interactions/compounds are: H:H eclipsing – 4.0 kJ/mol, 1.0 kcal/mol, H: CH3 eclipsing – 5.8 kJ/mol, 1.4 kcal/mol.
The strain energy for alkanes interaction is as follows: H : H eclipsing - 4.0 KJ/mol or 1.0 Kcal/mol: CH3 eclipsing - 5.8 KJ/mol or 1.4 Kcal/mol CH3 : CH3 eclipsing - 11.0 KJ/mol or 2.6 Kcal/mol.
Gauche butane - 3.8 KJ/mol or 0.9 Kcal/mol Cyclopropane - 115 KJ/mol or 27.5 Kcal/molCyclobutane - 110 KJ/mol or 26.3 Kcal/molCyclopentane - 26.0 KJ/mol or 6.2 Kcal/molCycloheptane - 26.2 KJ/mol or 6.3 Kcal/molCyclooctane - 40.5 KJ/mol or 9.7 Kcal/moL. The energy units are written in uppercase. The difference between kj/mol and kcal/mol is that kj/mol is the SI unit of energy, and kcal/mol is the cgs unit of energy.
Read more about energy:
https://brainly.com/question/13881533
#SPJ11
PLEASE HELP THIS IS URGENT
Answers
Answer:
in the first box the answer will be=37.2
and in the second box= 22.4
what is the name of a molecule that differs in the number of electrons, but has the same number of protons?
Answers
A molecule with the same number of protons but different number of electrons is known as an isotope.
Isotopes are atoms of the same element with different numbers of neutrons, and thus different atomic mass.
Isotopes form when an atom gains or loses an electron, resulting in an atom with the same number of protons but a different number of electrons.
Atoms of the same element with different numbers of neutrons are known as isotopes. When an atom gains or loses an electron, the number of protons stays the same but the number of electrons changes.
This change in the number of electrons alters the properties of the atom, and the different forms of the same element are known as isotopes.
The number of electrons in an atom determines how an atom interacts with other atoms.
Atoms with an even number of electrons tend to interact with each other in a more stable manner than atoms with an odd number of electrons.
This is why isotopes of elements that can exist in different forms have different chemical properties.
The isotopes of an element have different weights, and this is the result of the different numbers of neutrons. Isotopes can also have different nuclear properties and different radioactive properties.
In summary, an isotope is a molecule that differs in the number of electrons, but has the same number of protons.
This change in the number of electrons alters the properties of the atom, such as its chemical and nuclear properties.
to know more about isotope refer here:
https://brainly.com/question/11680817#
#SPJ11
why is aluminium brass used to make door handles instead of pure copper ?
Answers
Brass Door Plates and door knobs have been used on doors for centuries. Solid Brass fixtures fell out of favour only during the last couple of decades of the 20th century, largely because of the need regularly polish the metal to maintain its shine. Manufacturers began to lacquer (or varnish) their brass products to maintain a bright yellow finish, and lacquer eventually began to be regarded as gaudy by some people. This led to Brass falling by the wayside in favour of ‘cleaner’ looking metals, such as stainless steel, aluminium, and polished chrome.Several scientific studies have recently been published which suggest that Brass handles, door plates, door knobs and handrails should be brought back into regular use in public buildings, to help combat bacteria and germs, amazingly including hospital superbugs such as E-coli and MRSA Copper is the predominant metal used in the mixing of Brass Alloy. This means that copper-based metals such as brass, can prevent bacteria from spreading, and even completely destroy germs and bacteria.Researchers found that plastic and stainless steel surfaces, which are now the most widely used surfaces in hospitals and public buildings, allow bacteria to survive and spread when people touch them. The especially nasty viruses Norovirus and C-Diff can survive for much longer. Norovirus can survive for several weeks, while in one study C-Diff was shown to survive for an incredible five months.Researchers found that copper-based alloy surfaces have the ability to destroy a wide range of microbes and bacteria relatively rapidly - often within two hours or less. Several studies found that if touch surfaces are made with copper-based alloys, the reduced transmission of disease-causing bacteria can reduce patient infections in hospitals by as much as 58%.Copper has even been shown to be very effective at exterminating the much-dreaded hospital ‘superbug’ MRSA. In tests sponsored by the Copper Development Association, a grouping of 100 million MSRA bacteria atrophied and died in a just 90 minutes, when placed on a copper surface at room temperature. The same study found that the same number of MSRA bacteria on both steel and aluminium surfaces actually increased over time. On looking at these figures, many scientists have concluded that the installation of copper-based fixtures such as taps, light switches, door handles, door knobs, pull handles, and push plates in areas such as hospitals could save thousands of lives each year.In research published in the journal Molecular Genetics of Bacteria Professor Keevil wrote: “There are a lot of bugs on our hands that we are spreading around by touching surfaces. In a public building or mass transport, surfaces cannot be cleaned for long periods of time… Until relatively recently brass was a relatively commonly used surface. On stainless steel surfaces these bacteria can survive for weeks, but on copper surfaces they die within minutes… We live in this new world of stainless steel and plastic, but perhaps we should go back to using brass more instead.”In addition to direct contact killing of bacteria and harmful microbes, amazingly Copper surfaces have been found to exude an antimicrobial 'halo' effect on surrounding non-copper surfaces. Research in the intensive care unit a Hospital in Greece found that other surfaces up to 50 centimetres from copper surfaces experienced 70% microbial reduction, compared to the same surfaces with no proximity to copper-based materials. The ‘Halo’ effect was also observed in trials at a U.S. clinic in 2010. This amazing effect demonstrates just how powerful copper is as a weapon against bacteria.Since this research has come to light, historians have pointed out that some ancient civilizations were aware of the antimicrobial properties of copper, thousands of years before the concept of microbes became understood by modern science. In addition to the use of copper medicinal preparations, ancient people observed that water stored in copper vessels was of better quality than water contained or transported in other materials, as no slime can form on copper surfaces. In addition, the healing power of copper was recognized by the Aztecs and the Ancient Egyptians to sterilize wounds, drinking water, and used the metal to treat skin conditions.Several scientific studies suggest that copper surfaces affect bacteria in two ways. The first step is a direct interaction between the surface and the bacteria’s outer membrane, causing this to rupture. The second step involves the holes in the outer membrane, through which the cell loses essential nutrients and waterWhen the cells main defense membrane is breached, a stream of copper ions can enter the cell. Copper literally overwhelms the inside of the cell and obstructs the cell metabolism. It binds to the cell’s enzymes, causing its essential activity to stop. After this process, the bacteria can no longer "breathe", "eat" or "digest" and is thus essentially dead.
g a positive benedict's test is indicated by the formation of which of the following? a. cu2o b. cu c. cu2 d. metallic mirror
Answers
The formation of a reddish brown color precipitate ([tex]Cu_{2}O[/tex]) is an indication of a positive Benedict's test. The Benedict's test is a chemical test used to identify the presence of reducing sugars, and the formation of brick-red precipitate, indicates a positive result.
The substances tested are usually aqueous solutions of simple sugars (like glucose) or complex carbohydrates (like starch). The result is indicated by the formation of copper oxide (tex]Cu_{2}O[/tex]) or copper (Cu) in a reaction with a solution of Benedict's reagent.
A positive Benedict's test is indicated by the formation of [tex]Cu_{2}O[/tex].The Benedict's test is a semi-quantitative method that is commonly used to detect the presence of reducing sugars in a solution. The copper (II) ions in the Benedict's solution are reduced to copper (I) ions when they react with the reducing sugars, resulting in a precipitate. The copper (I) oxide ([tex]Cu_{2}O[/tex]) precipitate, which is reddish-brown in color, forms when there is a positive Benedict's test reaction.
The correct option is A. [tex]Cu_{2}O[/tex].
For more questions related to Benedict's test.
https://brainly.com/question/25800056
#SPJ11
review the terms and their definition in the mini glossary. write a sentence that shows your understanding of one of the properties of metals.
Answers
Metals are ductile and malleable. Metals are efficient heat and wires. Metals could be treated but are lustrous (sparkly). Iron are solids when room temperature (except arsenic, which is fluid). Metals are resilient and powerful.
What does the term "metals " mean?Metal is a solid substance that is hard, lustrous, ductile, fusible, and ductile and carries heat and electricity. Materials that have a propensity to give electrons include metals. They have an electropositive makeup.
Why are metals so valuable?Metals are excellent building materials. Strength, hardness, and rigidity are just a few of the qualities that metals possess. Metals may be cooked and formed into anything, from a little paperclip to an enormous aircraft. They are important for generators in cooking utensils due to their outstanding thermal and electrical conductors.
To know more about lustrous visit:
https://brainly.com/question/14872281
#SPJ1
A container has a pressure of 5,64 atm and a volume of 26.0 L. The volume of the container was decreased until the pressure was 9:17 atm. What is the new volume?
Answers
The new volume of the container after the decrease in the former volume would be = 15.99L
How to calculate the final volume of the container?The initial pressure in the container (P1) = 5.64 atm
The final pressure in the container (P2) = 9.17 atm
The initial volume of the container = 26.0 L
The final volume of the container = ?
to
Using Boyle's law formula;
P1V1 = P2V2
5.64 ×26.0 = 9.17 ×V2
make V2 the subject of formula;
V2 = 5.64×26/9.17
V2 = 146.64/9.17
V2 = 15.99L
Therefore, the new volume of the container is 15.99L which decreased due to increase in pressure.
Learn more about volume here:
https://brainly.com/question/27710307
#SPJ1
what is the product of the reaction sequence below 2 methyl 1 hexanol 2 methyl 2 hexanol 1 heptanol 2 heptanol
Answers
The product of the reaction sequence below is
2-methyl-1-hexanol
and 2-methyl-2-hexanol.
The reaction sequence starts with 2-methyl-1-hexanol and 2-methyl-2-hexanol. These molecules react to form a
carbocation
intermediate, which is then attacked by a molecule of water. This forms a tertiary alcohol,
1-heptanol
. Finally, 1-heptanol undergoes dehydration to form a double bond, forming 2-heptanol.
The overall reaction is an example of a
hydration
reaction, in which a molecule of water is added to the substrate in order to produce an alcohol. This reaction is catalyzed by an acid, such as sulfuric acid. The acid helps to activate the carbocation intermediate, allowing it to be attacked by the nucleophilic water molecule.
The reaction is an example of a Markovnikov addition, in which the hydrogen atom of the water molecule is added to the carbon with the most hydrogens already attached. In this reaction, the hydrogen is added to the primary carbon of the alkene, and the double bond shifts to the secondary carbon. This forms the tertiary alcohol, 1-heptanol.
Finally, 1-heptanol undergoes dehydration to form a double bond, forming 2-heptanol. This is an example of an E1 elimination reaction, in which the alcohol is protonated and then the proton is abstracted by a base, forming the alkene.
In conclusion, the product of the reaction sequence is 2-methyl-1-hexanol and 2-methyl-2-hexanol.
To know more about
hydration
reaction
click on below link:
https://brainly.com/question/12951675
#SPJ11
under standard conditions (298 k and 1 atm), which statement is true? refer to the constants for thermodynamic properties under standard conditions. a. diamond converts to graphite spontaneously b. graphite converts to diamond spontaneously c. none of the above
Answers
Under standard conditions (298 K and 1 atm), neither statement is true.
Diamond and graphite are both forms of carbon and are in a state of equilibrium under standard conditions. This means that neither diamond nor graphite will spontaneously convert to the other form.
Therefore, the correct answer is option (c): none of the above.
For more questions like thermodynamic visit the link below:
The thermodynamic equilibrium constant In a chemical equilibrium, K is the appropriate quotient of species activities. Under normal temperatures and pressures, an activity cannot be very many orders of magnitude more than 1.
The definition of thermodynamic properties is "system characteristics that can specify the state of the system." Certain constants, like R, are not attributes since they do not describe the state of a system.
Thermodynamics states that the conversion of diamond to graphite occurs spontaneously and is favourable. Yet, this reaction moves extremely slowly because kinetics, not thermodynamics, regulates it. As a result, diamond is thermodynamically unstable but kinetically stable.
https://brainly.com/question/29508731
#SPJ11
calculate the number of moles when 50g of copper (II) sulfate crystals, CuSO4.5H2O
Answers
Explanation:
To calculate the number of moles of copper (II) sulfate crystals (CuSO4.5H2O) in 50g, we first need to find the molar mass of CuSO4.5H2O.
The molar mass of CuSO4 is:
Cu: 63.55 g/mol
S: 32.06 g/mol
O (4 atoms): 15.99 g/mol x 4 = 63.96 g/mol
H2O (5 molecules): 18.02 g/mol x 5 = 90.10 g/mol
Therefore, the molar mass of CuSO4.5H2O is:
Molar mass = (63.55 + 32.06 + 63.96 + 90.10) g/mol
= 249.67 g/mol
Next, we can use the formula:
number of moles = mass / molar mass
where mass is the given mass of CuSO4.5H2O, and molar mass is the value we just calculated.
Substituting the values, we get:
number of moles = 50 g / 249.67 g/mol
= 0.2002 mol (rounded to four significant figures)
Therefore, 50g of copper (II) sulfate crystals contain 0.2002 moles of CuSO4.5H2O
fumes of iodine are produced when potassium iodide is oxidized by concentrated sulfuric acid.Write a correct equation of reaction.
Answers
Chemical equation: The reaction can be described by: KI + H2SO4 -> K2SO4 + H2O -> I2 The potassium iodide (KI), which contains iodide ions (I-), is oxidised by the sulfuric acid to produce molecular iodine in this reaction (I2).
What occurs when concentrated sulphuric acid and potassium iodide react?Deep violet vapours with a strong scent would develop when concentrated sulfuric acid was added drop by drop to solid potassium iodide. If concentrated sulfuric acid is gradually introduced to solid potassium chloride, it will not result in the formation of these violet fumes.
In the presence of diluted sulphuric acid, what colour results from the reaction of iodine with potassium iodide?Iodide ions are created when sodium sulphite and potassium iodate combine, and this process also results in the oxidation of iodide ions in an acidic medium.
To know more about iodine visit:-
https://brainly.com/question/16867213
#SPJ9
which electronegativities do you subtract to find out if a bond is polar or not in a 3 element compoudn
Answers
Answer: To find out if a bond is polar or not in a 3 element compound, you subtract the electronegativities of the two atoms forming the bond.
The difference in electronegativity values will help determine the polarity of the bond. If the difference is large enough, the bond will be polar, and if the difference is small or non-existent, the bond will be nonpolar.
What is electronegativity?
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is the property of an atom that shows how strongly it pulls electrons towards itself. When atoms bond with each other, the electrons involved in bonding are not always shared equally.
What is a polar bond?
A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally shared. This results in a slight positive charge on one end of the molecule and a slight negative charge on the other end. In other words, one end of the molecule is more electronegative than the other.
What is a nonpolar bond?
A nonpolar bond is a covalent bond between two atoms where the electrons forming the bond are shared equally. This results in no separation of charge across the molecule.
What are the rules for identifying polar or nonpolar bonds in 3 element compounds?
To identify whether a bond is polar or nonpolar, subtract the electronegativities of the two atoms forming the bond. If the difference is less than 0.5, then the bond is nonpolar. If the difference is between 0.5 and 1.7, then the bond is polar. If the difference is greater than 1.7, then the bond is considered ionic.
Learn more about polar bond here:
https://brainly.com/question/10777799#
#SPJ11
the reaction rate is measured as -2.6 m ch4/s. determine the rate of appearance of co2 and the rate of appearance of h2o. explain how you arrived at your answers.
Answers
The stoichiometric coefficients can be used to connect the rates at which certain components appear or vanish in the reactions that are described. Applying this to the responses given,
1. Reaction: N₂(g) + 3H₂(g) → 2NH₃(g)
Given reaction rate: 0.032 M NH₃/s
According to the stoichiometry:
For every 1 mole of N₂, 2 moles of NH₃ are produced.For every 3 moles of H₂, 2 moles of NH₃ are produced.Therefore, the rate of disappearance of N₂ is given by:
Rate of disappearance of N₂ = (0.032 M NH₃/s) * (1 mol N₂ / 2 mol NH₃)
= 0.016 M N₂/s.
Similarly, the rate of disappearance of H₂ is given by:
Rate of disappearance of H₂ = (0.032 M NH₃/s) * (3 mol H₂ / 2 mol NH₃)
= 0.048 M H₂/s.
2. Reaction: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
Given reaction rate: 2.6 M CH₄/s
According to the stoichiometry:
For every 1 mole of CH₄, 1 mole of CO₂ is produced.For every 1 mole of CH₄, 2 moles of H₂O are produced.
Therefore, the rate of appearance of CO₂ is given by:
Rate of appearance of CO₂ = (2.6 M CH₄/s) * (1 mol CO₂ / 1 mol CH₄) = 2.6 M CO₂/s.
Similarly, the rate of appearance of H₂O is given by:
Rate of appearance of H₂O = (2.6 M CH₄/s) * (2 mol H₂O / 1 mol CH₄)
= 5.2 M H₂O/s.
Thus, we used the stoichiometric coefficients to relate the rates of different components in the reactions.
For more details regarding stoichiometric coefficients, visit:
https://brainly.com/question/32088573
#SPJ12
Your question seems incomplete, the probable complete question is:
In the lab activity, the reaction rate was determined by the appearance of a product. However, the reaction rate can also be determined by the disappearance of a reactant. --aructi or Rate-a[Reactant] In each situation below, you are given a rate measured by the appearance of one component of the reaction and are asked to predict the rate of appearance or disappearance of another component, based on logic and stoichiometric relationships. For example, if the reaction is as follows: A +2B Products For every mole of A that is used, 2 moles of B are used so the rate of disappearance of B is twice the rate of the disappearance of A. This may be expressed as: Rate =-=-N2(g) + 3H2 (g) ? 2NH3(g) The reaction rate is measured as 0.032 M NHy/s. Determine the rate of disappearance of N2 and the rate of disappearance H2. Explain how you arrived at your answers. CH4(g)+202(g) -CO2(g)+2H,0(8) The reaction rate is measured as 2.6 M CH/s. Determine the rate of appearance of CO2 and the rate of appearance of H20. Explain how you arrived at your answers
is the green salt more or less soluble in hot than in cold water? how did you utilize this information during the experiment
Answers
The green salt is less soluble in hot water than in cold water. During the experiment, this information can be utilized to adjust the temperature of the water to control the solubility of the salt.
The quantity of a substance that can dissolve in a particular solvent is known as solubility. Solubility is dependent on the properties of the solvent, the solute, and the solution. Temperature, pressure, and, in the case of ionically conducting solvents, electric fields also play a role.
Solubility is expressed as the maximum amount of solute that may be dissolved in a particular quantity of solvent at a specific temperature to create a saturated solution. Solubility of green salt, Green salt, also known as copper(II) acetate, is a substance with a solubility of 1.6 g/100 mL in cold water and 1.8 g/100 mL in hot water.
This means that green salt is more soluble in hot water than in cold water, according to the values given in the question. During the experiment, this information on the solubility of green salt in hot and cold water could be utilized to control the solubility of the salt.
Adjusting the temperature of the water to make it colder would increase the solubility of green salt in it, while adjusting the temperature of the water to make it hotter would decrease the solubility of green salt in it.
To know more about solubility, refer here:
https://brainly.com/question/31043941#
#SPJ11
which separation technique would be the best method to separate a 1:1 mixture of aniline and ethylbenzene?
Answers
The best method to separate a 1:1 mixture of aniline and ethylbenzene is through
distillation
.
Distillation is a process that involves heating the mixture to its boiling point, which causes the components to vaporize. As the vapors cool and condense, the liquid components will separate into their pure forms.
Since the boiling points of aniline and
ethylbenzene
differ significantly Aniline boiling point: 184°C; Ethylbenzene boiling point: 135°C.
The process of distillation involves heating the mixture in a distillation apparatus.
As the temperature increases, the vaporized components of the mixture will travel up a condenser and then be collected separately in two separate flasks.
During this process,
aniline
will be the first component to vaporize and travel up the condenser, while ethylbenzene will follow suit.
The two components will condense in their respective flasks and can then be collected and isolated.
In conclusion,
Distillation is the best method to separate a 1:1 mixture of aniline and ethylbenzene due to the fact that it utilizes their differences in boiling points to allow for the collection of the two components in their pure forms.
This is achieved by heating the mixture in a distillation apparatus and condensing the vapors in two separate flasks.
to know more about
distillation
refer here:
https://brainly.com/question/29037176#
#SPJ11
as the temperature increases, the rate of enzymatic reactions can ; however, at extremely high temperatures (95c) the rate will dramatically due to .
Answers
As the temperature increases, the rate of enzymatic reactions generally increases as well, because the molecules have more kinetic energy and collide more frequently.
What are enzymes?Enzymes are proteins with specific three-dimensional shapes that are critical to their function. At high temperatures, the increased kinetic energy can disrupt the weak forces that hold the protein's structure together, causing the enzyme to lose its shape and become denatured. Denatured enzymes can no longer bind to substrates, and the rate of enzymatic reactions will drop sharply.
The temperature at which an enzyme denatures depends on the specific enzyme and its optimal temperature range. Some enzymes are adapted to function at very high temperatures, such as those found in thermophilic bacteria that live in hot springs or hydrothermal vents.
However, most enzymes have a more narrow temperature range within which they can function optimally, and extreme temperatures can cause irreversible damage to the enzyme structure.
Learn more about enzymatic reaction here: https://brainly.com/question/1596855
#SPJ1
Problem 11.9 Starting with acetyl chloride; what neutral nucleophile would you use to make each of the following compounds? Part € CH; Draw the molecule on the canvas by_ toolbars_
Answers
To make each of the following compounds starting with acetyl chloride, neutral nucleophiles to be used are:Compound Part € CH: To prepare this compound starting with acetyl chloride, neutral nucleophile, ethylamine (C2H5NH2) is used. Here's how you can prepare it.
neutral nucleophile to be used to prepare Compound Part € CH is C2H5NH2. You can prepare it by reacting acetyl chloride with ethylamine. The reaction of acetyl chloride with ethylamine produces CH3C(O)NHC2H5 by releasing
hydrogen chloride gas. Parts a, b, c: The compounds given in parts a, b, and c are carboxylic acids. To prepare these carboxylic acids starting with acetyl chloride, neutral nucleophiles to be used are NaOH, H2O, and CH3COOH,
respectively. Here's how you can prepare these compounds:Part a: CH3COCl + NaOH → CH3COONa + HClPart b: CH3COCl + H2O → CH3COOH + HClPart c: CH3COCl + CH3COOH → CH3COOCH3 + HCl
Thus, the neutral nucleophiles to be used to prepare Part a, b, and c are NaOH, H2O, and CH3COOH, respectively. You can prepare them by reacting acetyl chloride with NaOH, H2O, and CH3COOH, respectively. The reactions of acetyl chloride with NaOH, H2O, and CH3COOH produce CH3COONa, CH3COOH, and CH3COOCH3, respectively, by releasing hydrogen chloride gas.
For more similar questions on topic neutral nucleophile
brainly.com/question/14052597
#SPJ11
what occurs when aqueous silver nitrate, a g n o 3 , reacts with aqueous potassium sulfate, k 2 s o 4 ?
Answers
Answer: A white precipitate of silver sulfate (Ag2SO4) is formed.
Explanation:
When aqueous silver nitrate (AgNO3) reacts with aqueous potassium sulfate (K2SO4), a double displacement reaction occurs. The cations and anions of the two compounds switch places to form two new compounds, which are potassium nitrate (KNO3) and silver sulfate (Ag2SO4).
AgNO3 + K2SO4 → Ag2SO4 + 2KNO3
The insoluble product of this reaction is silver sulfate (Ag2SO4), which appears as a white precipitate. This reaction is commonly used to detect the presence of sulfate ions in solution, as the formation of the silver sulfate precipitate confirms the presence of sulfate ions.
francine added 3.0 ml of 4.0 m koh to 6.0 ml of 0.30 m hbr. determine whether the resulting mixture is acidic, basic, or neutral.
Answers
The resulting mixture is basic because the KOH is a strong base and the HBr is a weak acid.
To determine whether the resulting mixture is acidic, basic, or neutral, the concentration of hydroxide ions (OH-) and hydronium ions (H+) in the solution is compared. Since KOH is a base and HBr is an acid, it is essential to determine the net ionic equation. Here's the balanced chemical equation:
KOH(aq) + HBr(aq) → KBr(aq) + H2O(l)
Since the balanced equation represents a neutralization reaction, the concentration of OH- and H+ can be determined based on the reaction. Therefore, in the reaction, the number of OH- ions will be equal to the number of H+ ions.In the above reaction, 1 mole of KOH reacts with 1 mole of HBr to form 1 mole of KBr and 1 mole of water. As a result, the mole of KOH added in the reaction is;
Number of moles of KOH = volume × concentration= 3.0 ml × (4.0 mol/L)/1000 mL/L= 0.012 mol
The mole of HBr reacted in the reaction is:
Number of moles of HBr = volume × concentration= 6.0 mL × (0.30 mol/L)/1000 mL/L= 0.0018 mol
Therefore, the number of moles of HBr is less than the number of moles of KOH. Since KOH is a base and HBr is an acid, the net ionic equation is as follows:
H+ + OH- → H2O
In this reaction, the number of OH- ions is greater than the number of H+ ions; therefore, the solution is basic. Therefore, the resulting mixture is basic.
More on acid/bases: https://brainly.com/question/31030349
#SPJ11
when millerite, an ore containing solid nis , is roasted in the presence of oxygen, sulfur dioxide gas and a solid oxide of nickel are produced. in the reaction, nickel does not change oxidation state.enter the balanced chemical equation for the metallurgical reaction. include physical states.
Answers
The balanced chemical equation for the metallurgical reaction when millerite, an ore containing solid NiS, is roasted in the presence of oxygen can be given as;
2NiS(s) + 3O2(g) → 2NiO(s) + 2SO2(g)
The physical states in this equation are: NiS (s), O2 (g), NiO (s), and SO2 (g).
Explanation:
Millerite is a nickel sulfide mineral that consists of nickel and sulfur. When millerite is roasted in the presence of oxygen, it forms nickel oxide (NiO) and sulfur dioxide (SO2).
The oxidation state of nickel doesn't change because it's only reacting with oxygen.
NiS(s) + O2(g) → NiO(s) + SO2(g)
The balanced chemical equation for the metallurgical reaction when millerite, an ore containing solid NiS, is roasted in the presence of oxygen can be given as;2NiS(s) + 3O2(g) → 2NiO(s) + 2SO2(g)
The physical states in this equation are
NiS (s), O2 (g), NiO (s), and SO2 (g).
To know more about the millerite https://brainly.com/question/29856128
#SPJ11